Abstract
Introduction: Individuals with congenital afibrinogenemia can experience frequent and/or severe bleeding episodes (BE). Human fibrinogen concentrate (HFC) can correct the hemostatic defect and arrest bleeding. We investigated the efficacy, safety and pharmacokinetics (PK) of a new highly purified, double virus-inactivated HFC (Fibryga, Octapharma) in adolescent patients.
Methods: Data were analyzed from two multinational, prospective, open-label studies. FORMA-01 studied the PK of the new HFC vs. comparator (Haemocomplettan P), as well as surrogate efficacy and safety, after single infusion of 70 mg/kg. Surrogate efficacy was defined as thromboelastometric maximum clot firmness (MCF). FORMA-02 was a Phase 3 study in which the primary endpoint was the hemostatic efficacy of the new HFC for on-demand treatment of the first bleeding event. A 4-point objective scale, which was adjudicated by an Independent Data Monitoring and Endpoint Adjudication Committee (IDMEAC), was used for evaluation of efficacy. In addition, MCF and safety were evaluated for all BEs that occurred during the study period.
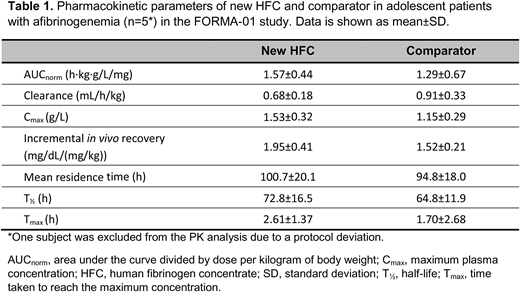
Results: Data were available for 12 patients aged 12-17 years with afibrinogenemia. FORMA-01 included 5 patients aged 12-17 years (1 was excluded due to a protocol deviation); PK data for new HFC vs. comparator are shown in Table 1. For comparison, in adults from the same study AUCnorm was also larger and clearance slower for new HFC vs. comparator (both p=0.0027). At 1 h following infusion of new HFC or comparator, mean±SD MCF (n=6) increased from 0 mm to 9.0±2.0 and 8.8±2.6 mm, respectively (9.9±3.3 and 10.4±4.9 mm in adults).
In FORMA-02, 6 adolescent patients received new HFC individually dosed for treatment of a BE. Hemostatic efficacy for treatment of the first BE was rated as excellent for all patients (success: 100%; 90% CI: 0.655-1.000). When taking all BEs into account (n=23), efficacy was again classed as excellent for all patients. Mean MCF increased by 4.83±0.98 mm from baseline to 1 h after first infusion (7.06±3.36 mm in adults). There were no related serious adverse events, severe allergic, hypersensitivity reactions, or thromboembolic events, and no inhibitory anti-fibrinogen antibodies were detected.
Conclusions: This analysis of data from two prospective studies demonstrated favorable efficacy, safety, and PK parameters of the new HFC in adolescents with afibrinogenemia. PK parameters were broadly comparable with those of the comparator HFC. After infusion of the new HFC, MCF increased statistically significantly and hemostatic efficacy was rated excellent in all patients. No safety concerns relating to the new HFC were identified in these study patients.
Peyvandi:Ablynx: Other: Member of Advisory Board, Speakers Bureau; Ablynx: Other: Member of Advisory Board, Speakers Bureau; Octapharma US: Honoraria; Ablynx: Other: Member of Advisory Board, Speakers Bureau; Octapharma US: Honoraria; Ablynx: Other: Member of Advisory Board, Speakers Bureau; Ablynx: Other: Member of Advisory Board, Speakers Bureau; Kedrion: Consultancy; Kedrion: Consultancy; Kedrion: Consultancy; Kedrion: Consultancy; Grifols: Speakers Bureau; Kedrion: Consultancy; Grifols: Speakers Bureau; Grifols: Speakers Bureau; Novo Nordisk: Speakers Bureau; Novo Nordisk: Speakers Bureau; Grifols: Speakers Bureau; Grifols: Speakers Bureau; Roche: Speakers Bureau; Novo Nordisk: Speakers Bureau; Roche: Speakers Bureau; Novo Nordisk: Speakers Bureau; Novo Nordisk: Speakers Bureau; Roche: Speakers Bureau; Shire: Speakers Bureau; Shire: Speakers Bureau; Roche: Speakers Bureau; Roche: Speakers Bureau; Shire: Speakers Bureau; Sobi: Speakers Bureau; Shire: Speakers Bureau; Sobi: Speakers Bureau; Sobi: Speakers Bureau; Shire: Speakers Bureau; Octapharma US: Honoraria; Sobi: Speakers Bureau; Sobi: Speakers Bureau; Octapharma US: Honoraria; Octapharma US: Honoraria. Schwartz:Octapharma US: Employment. Knaub:Octapharma AG: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal